
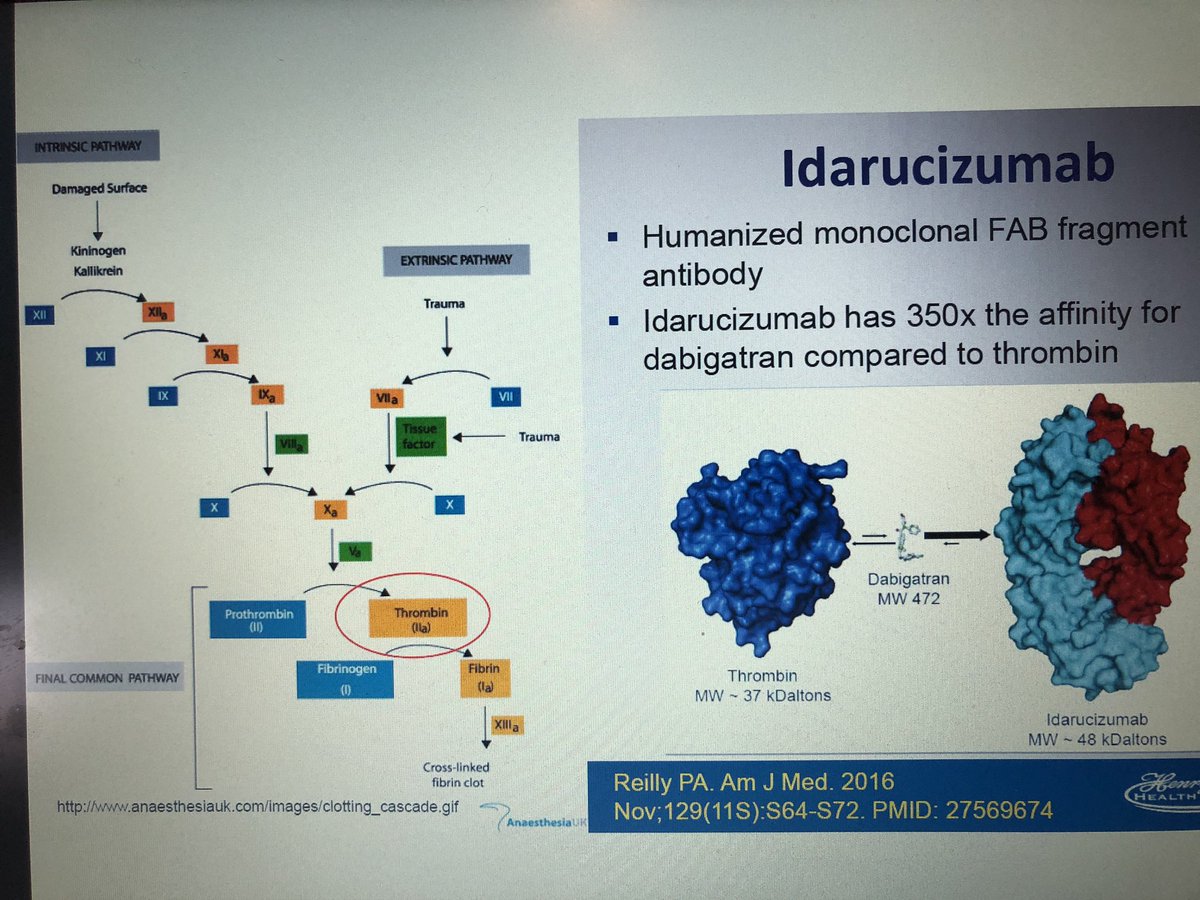
Primary outcome: median maximum percentage reversal within 4 hours after the administration of idarucizumab was 100% (95% CI, 100 to 100).95% of patients were taking dabigatran for atrial fibrillation.2015) and this study now represents the full analysis of a cohort of patients given Idarucizumab for the reversal of dabigatran in “uncontrollable or life-threatening” bleeding. Initial data from the only clinical study of the reversal agent was released in 2015 ( Pollack, Reilly et al. The drug is marketed in the US as Pradaxa, manufactured by Boehringer Ingelheim who subsequently designed and marketed the only available reversal agent for dabigatran, a monoclonal antigen-binding fragment (“Fab”) Idarucizumab, approved in 2015 by the FDA.

Previously there were no reversal agents available for dabigatran and monitoring of the drug is considered unreliable using standard lab tests. Dabigatran blocks the last stages of the coagulation cascade, specifically the cleavage of fibrinogen into fibrin, activation of platelets, and stabilization of forming clots. It is now routinely prescribed for the treatment of non-valvular atrial fibrillation, venous thromboembolism (VTE) and post-surgical prophylaxis. Dabigatran is an oral direct thrombin inhibitor, touted as non-inferior to Warfarin (though there is some debate about its safety ( Yao, Abraham et al.


 0 kommentar(er)
0 kommentar(er)
